The least basic aqueous solution among the following is:
1.
2.
3.
4.
Which one of the following on reduction with LiAlH4 yields a secondary amine ?
[2007]
1. Methyl isocyanide
2. Acetamide
3. Methyl cyanide
4. Nitroethane
The IUPAC name of the compound having formula,
is :
1. 3-Aminohydroxypropionic acid
2. 2-Amino-propan-3-oic acid
3. Amino hydroxy propionic acid
4. 2-Amino-3-hydroxy propanoic acid
In the reaction,
the term Y is
[1999]
1. Acetone
2. Ethanamine
3. Acetaldehyde
4. Dimethyl amine
RMgX on reacting with cyanogen chloride gives:
1. R-NC
2. R-Cl
3. R-CN
4. None of the above
X and Y in the given reaction are:

1.
2.
3.
4.
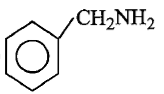
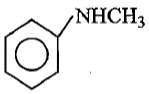
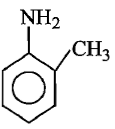
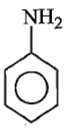
Which of the following is the strongest base ?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
1. 
2. 
3. 
4. 
Primary, secondary and tertiary nitroalkanes can be identifier by the action of:
1.
2.
3.
4. none of these
Consider the following sequence of reactions
Compound[A][B]
The compound [A] is
1. CH3CH2CN
2. CH3NO2
3. CH3NC
4. CH3CN
Which of the following statements about primary amines is false ?
[2010]
1. Alkyl amines are stronger bases than aryl amines
2. Alkyl amines react with nitrous acid to produce alcohols
3. Aryl amines react with nitrous acid to produce phenols
4. Alkyl amines are stronger bases than ammonia






